
PCDC Data Portal
Data Access and Governance
What data are available for research?
All of the data currently within the PCDC are derived from completed clinical trials. The data are formatted according to disease-based data dictionaries. Disease-based data dictionaries drive the PCDC Data Portal by defining a standard format for representing data collected from various sources.
Currently data are available for the following diseases:
- Rhabdomyosarcoma
- Non-rhabdomyosarcoma
- Neuroblastoma
- Germ cell tumors
- Hodgkin lymphoma
- Acute myeloid leukemia
How were these data elements selected?
The data dictionaries were developed through a consensus-based process and are regularly revised and updated. While many data fields are described in the data dictionaries, some data fields may not be available for individual patients.
Are all the disease data dictionaries finalized?
Some data dictionaries have been formally adopted, while others are still in development. Please see the README and/or Background Information tab of each data dictionary for development and version information. The disease experts continue to identify data elements that would enrich the commons and support more research. We have a standard process for expanding the data dictionary or making changes when necessary.
May I use your data dictionary in my own work?
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
How do I explore data on the PCDC Data Portal?
Anyone can access the PCDC Data Portal by visiting https://portal.pedscommons.org/ and creating a free account. Information on how to use the PCDC Data Portal can be found on our documentation page. For technical support and assistance with the PCDC Data Portal, please contact us at pcdc_help@lists.uchicago.edu.
Data Exploration
With your account, you are able to explore data dictionaries, perform cohort exploration, and generate data visualizations for patient cohorts you define based on data filters. Participant line-level data are not accessible using these tools but can be requested through the data/project request process.
Acceptable Use
Outputs from the cohort discovery and visualization tools are intended only for the purpose of determining feasibility of a proposed research study and early hypothesis exploration following generally-acceptable statistical principles. These outputs are neither suitable nor permitted for dissemination or publication.
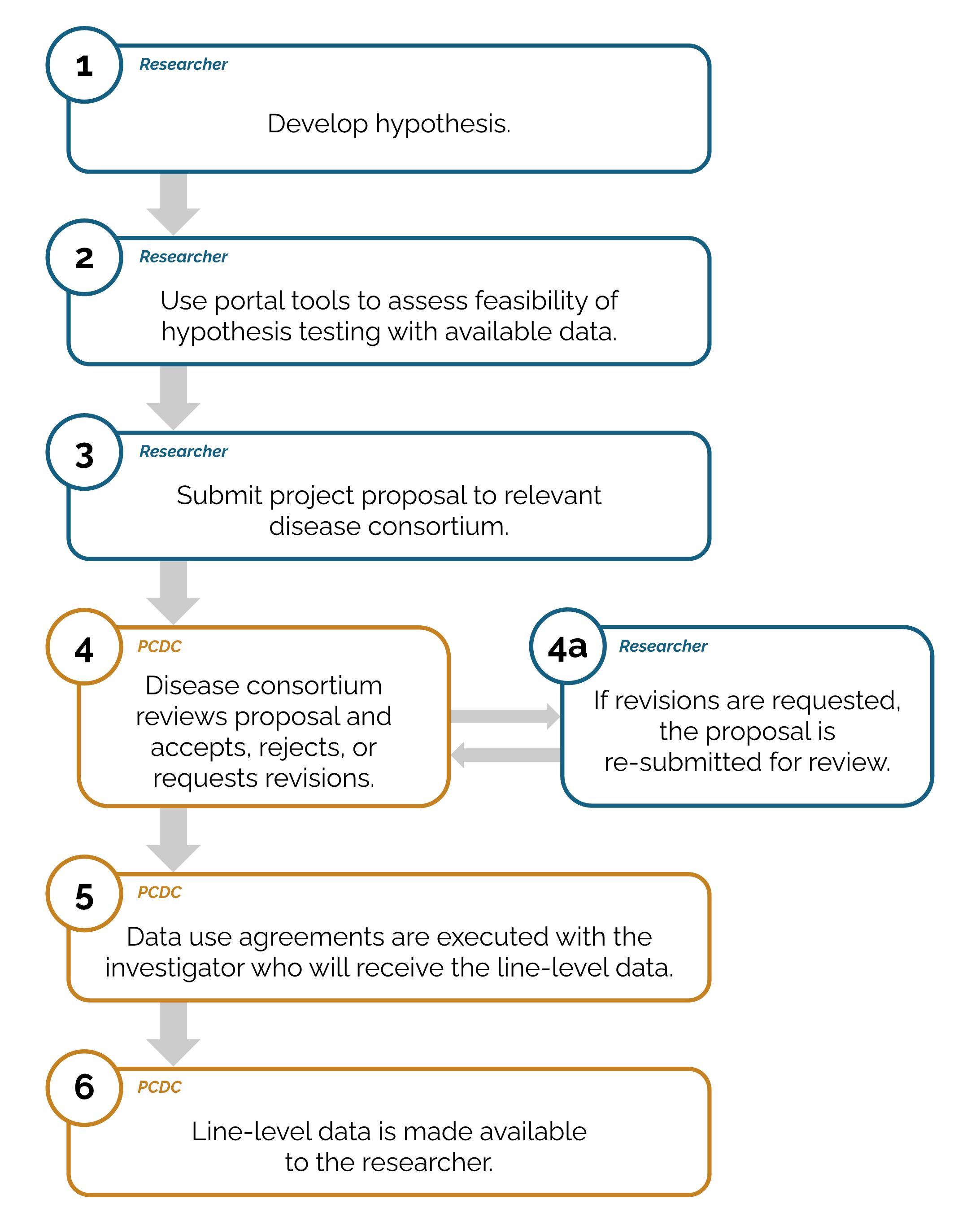
How do I request data from the PCDC Data Portal?
If you wish to perform research with data from the PCDC, your proposed project must undergo review and approval, and a data use agreement must be executed.
- You may request access to a specific dataset by following the project request process (see below).
- Following the approval of the project request, the PCDC legal team will work with you to put the appropriate legal agreements in place and then the PCDC will make the data available.
| Disease/Cancer | Consortium | Data Dictionary | Publication Policy | Project Request Form |
|---|---|---|---|---|
| ★ Rhabdomyosarcoma | INSTRuCT Contact: Suzi Birz |
RMS Link | Link | Link |
| ★ Neuroblastoma | INRG Contact: Suzi Birz |
NBL Link | Link | Link |
| ★ Germ cell tumors | MaGIC Contact: Lindsay Klosterkemper |
GCT Link | Link | Link |
| ★ Hodgkin lymphoma | NODAL Contact: Suzi Birz |
HL Link | Link | Link |
| ◕ Acute lymphoblastic leukemia | Contact: Enal Hindi | ALL Link | In development | In development |
| ★ Acute myeloid leukemia | INTERACT Contact: Enal Hindi |
AML Link | Link | Link |
| ◕ Cancer predisposition | C3P Contact: Nia Moyer |
C3P Link | In development | In development |
| ◕ Embryonal (CNS) | INSPiRE Contact: Maya Maric |
CNS Link | Link | Link |
| ◕ Ewing sarcoma (Bone) | HIBiSCus Contact: Kat Blumhardt |
EWS Link | Link | Link |
| ★ Non-rhabdomyosarcoma soft tissue sarcoma | INSTRuCT Contact: Suzi Birz |
NRSTS Link | Link | Link |
| ◕ Osteosarcoma (Bone) | HIBiSCus Contact: Kat Blumhardt |
OS Link | Link | Link |
| ◕ Retinoblastoma | Global REACH Contact: Maya Maric |
RB Link | In development | In development |
| ◕ Fanconi Anemia | FRIENDS Contact: Enal Hindi |
FRIENDS Link | Link | Link |
Key:
★: data available in the PCDC Data Portal
◕: data in progress
To request data from the PCDC, you will use the project request process defined by the individual disease consortium. For example, to request rhabdomyosarcoma data, you would follow the INSTRuCT process as detailed in the INSTRuCT Publication Policy. You can find Publication Policies and other detailed information for each disease with data available in the Data Portal through the links in the table above.
The PCDC disease consortium Executive Committee or designated group is responsible for review and approval of project requests associated with the consortium. You will receive feedback and next steps on your proposal following review. The review process will result in the project being accepted, rejected, or requiring revision. If revisions are requested, the project must be resubmitted to the disease consortium. If your project requires data from participants with more than one cancer type, each consortium will separately review your project proposal.
For consortium-specific questions on this process, please contact the consortium manager listed in the table above. For general questions, contact PCDC Program Manager Kat Blumhardt.